About us
Learn how GA4GH helps expand responsible genomic data use to benefit human health.
Learn how GA4GH helps expand responsible genomic data use to benefit human health.
Our Strategic Road Map defines strategies, standards, and policy frameworks to support responsible global use of genomic and related health data.
Discover how a meeting of 50 leaders in genomics and medicine led to an alliance uniting more than 5,000 individuals and organisations to benefit human health.
GA4GH Inc. is a not-for-profit organisation that supports the global GA4GH community.
The GA4GH Council, consisting of the Executive Committee, Strategic Leadership Committee, and Product Steering Committee, guides our collaborative, globe-spanning alliance.
The Funders Forum brings together organisations that offer both financial support and strategic guidance.
The EDI Advisory Group responds to issues raised in the GA4GH community, finding equitable, inclusive ways to build products that benefit diverse groups.
Distributed across a number of Host Institutions, our staff team supports the mission and operations of GA4GH.
Curious who we are? Meet the people and organisations across six continents who make up GA4GH.
More than 500 organisations connected to genomics — in healthcare, research, patient advocacy, industry, and beyond — have signed onto the mission and vision of GA4GH as Organisational Members.
These core Organisational Members are genomic data initiatives that have committed resources to guide GA4GH work and pilot our products.
This subset of Organisational Members whose networks or infrastructure align with GA4GH priorities has made a long-term commitment to engaging with our community.
Local and national organisations assign experts to spend at least 30% of their time building GA4GH products.
Anyone working in genomics and related fields is invited to participate in our inclusive community by creating and using new products.
Wondering what GA4GH does? Learn how we find and overcome challenges to expanding responsible genomic data use for the benefit of human health.
Study Groups define needs. Participants survey the landscape of the genomics and health community and determine whether GA4GH can help.
Work Streams create products. Community members join together to develop technical standards, policy frameworks, and policy tools that overcome hurdles to international genomic data use.
GIF solves problems. Organisations in the forum pilot GA4GH products in real-world situations. Along the way, they troubleshoot products, suggest updates, and flag additional needs.
GIF Projects are community-led initiatives that put GA4GH products into practice in real-world scenarios.
The GIF AMA programme produces events and resources to address implementation questions and challenges.
NIF finds challenges and opportunities in genomics at a global scale. National programmes meet to share best practices, avoid incompatabilities, and help translate genomics into benefits for human health.
Communities of Interest find challenges and opportunities in areas such as rare disease, cancer, and infectious disease. Participants pinpoint real-world problems that would benefit from broad data use.
The Technical Alignment Subcommittee (TASC) supports harmonisation, interoperability, and technical alignment across GA4GH products.
Find out what’s happening with up to the minute meeting schedules for the GA4GH community.
See all our products — always free and open-source. Do you work on cloud genomics, data discovery, user access, data security or regulatory policy and ethics? Need to represent genomic, phenotypic, or clinical data? We’ve got a solution for you.
All GA4GH standards, frameworks, and tools follow the Product Development and Approval Process before being officially adopted.
Learn how other organisations have implemented GA4GH products to solve real-world problems.
Help us transform the future of genomic data use! See how GA4GH can benefit you — whether you’re using our products, writing our standards, subscribing to a newsletter, or more.
Join our community! Explore opportunities to participate in or lead GA4GH activities.
Help create new global standards and frameworks for responsible genomic data use.
Align your organisation with the GA4GH mission and vision.
Want to advance both your career and responsible genomic data sharing at the same time? See our open leadership opportunities.
Join our international team and help us advance genomic data use for the benefit of human health.
Discover current opportunities to engage with GA4GH. Share feedback on our products, apply for volunteer leadership roles, and contribute your expertise to shape the future of genomic data sharing.
Solve real problems by aligning your organisation with the world’s genomics standards. We offer software dvelopers both customisable and out-of-the-box solutions to help you get started.
Learn more about upcoming GA4GH events. See reports and recordings from our past events.
Speak directly to the global genomics and health community while supporting GA4GH strategy.
Be the first to hear about the latest GA4GH products, upcoming meetings, new initiatives, and more.
Questions? We would love to hear from you.
Read news, stories, and insights from the forefront of genomic and clinical data use.
Publishes regular briefs exploring laws and regulations, including data protection laws, that impact genomic and related health data sharing
Translates findings from studies on public attitudes towards genomic data sharing into short blog posts, with a particular focus on policy implications
Attend an upcoming GA4GH event, or view meeting reports from past events.
See new projects, updates, and calls for support from the Work Streams.
Read academic papers coauthored by GA4GH contributors.
Listen to our podcast OmicsXchange, featuring discussions from leaders in the world of genomics, health, and data sharing.
Check out our videos, then subscribe to our YouTube channel for more content.
View the latest GA4GH updates, Genomics and Health News, Implementation Notes, GDPR Briefs, and more.
21 Aug 2024
Learn how the revised Product Development and Approval Process fosters consensus around GA4GH products through cooperation, consultation, and clear decision-making.

The Product Development and Approval Process of the Global Alliance for Genomics and Health (GA4GH) has undergone a significant update.
Approved by the GA4GH Product Steering Committee in 2023, the updated process — which applies to all GA4GH Work Streams other than Regulatory & Ethics — continues to ensure GA4GH products reflect broad consultation and consensus, both within GA4GH and with the wider genomics and health community.
Notably, the revised process includes clearly defined Problem Statements, explicit guidelines for appeals and required implementations, clearer processes around product updates, and new pre-planning and study phases before product development begins.
“The updated GA4GH Product Development and Approval Process aims to gauge community needs and avoid duplication of effort,” said Susan Fairley, the previous Chief Standards Officer (CSO) of GA4GH who spearheaded this initiative. “With this revised process, GA4GH hopes to strengthen measures for gathering feedback, building consensus, and communicating decisions at every step of product development. If you miss a meeting, for example, there should be no surprises.”
Voting rules have also been refined: now, Product Steering Committee members can delegate a nominee with the relevant domain knowledge to cast a vote on their behalf. More than half of the Product Steering Committee must vote, and at least 60% of those votes must be in support of the proposal for it to be approved.
Efforts to revamp the product approval process began after a 2021 survey indicated that the community sought improvements. Under Fairley’s leadership, GA4GH gathered extensive feedback and studied the processes of other standards development organisations. The new Product Development and Approval Process incorporates findings from that review.
Here, we walk you through the updated Product Development and Approval Process, explaining the steps necessary to take a new product or version from idea to approval. (Note that this article is for explanatory purposes only; all GA4GH contributors and participants must adhere to the actual text of the Product Development and Approval Process.)
Let’s say you’ve hit a roadblock in your work. You spot a standards-sized gap in the world of phenotyping or pharmacogenomics or data federation, and think, “GA4GH should do something about this.”
The Product Development and Approval Process is your guide to going through the process.
The process is guided by several core principles: cooperation and consultation are essential; products should be designed to work seamlessly together; and all efforts must align with the GA4GH Code of Ethics and Community Conduct. It’s crucial to note that at any point, parties can agree to stop development. Even if an idea is explored and then set aside, it’s considered a valuable part of the process.
All 33 points in the process combine into one continuous flow, with many points referring back to previous ones. Be sure to review the entire Product Development and Approval Process before proposing a new idea.
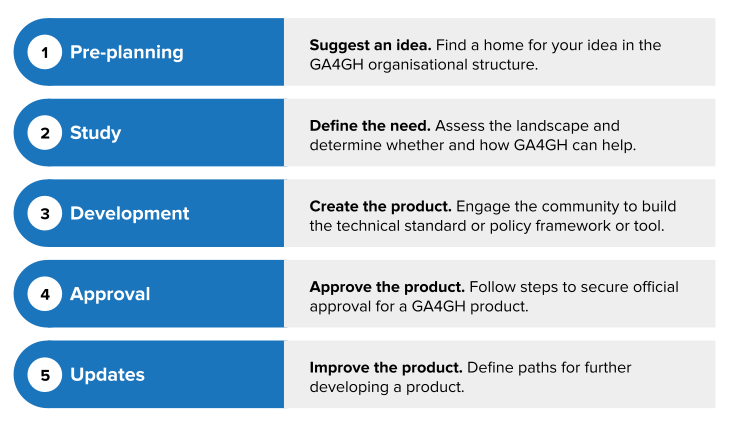
For the purpose of this basic introduction, we explain the process in five sections: pre-planning, study, development, approval, and updates.
When someone has an idea for a potential GA4GH product, the first step is pre-planning. Start here if you want to:
Pre-planning involves assessing the basics, such as: who is interested? Does this group duplicate efforts in the existing structure of GA4GH? Would any legal barriers prevent GA4GH from getting involved?
Interested individuals work with GA4GH staff to find their ideal home, whether in an existing GA4GH group or by assembling a new group. Once the group represents sufficiently broad geographic and domain areas, it can move into a study phase.
During its study phase, the group assesses the existing landscape, determining whether and how GA4GH can address the need under discussion.
Group members consult a broad swath of relevant people and institutions, including the GA4GH Regulatory & Ethics and Data Security Work Streams. Then they produce the following outputs:
They also assemble a Product Review Committee (PRC), which consists of three people with appropriate domain knowledge who are not part of the development work. The Product Review Committee considers the study outputs and determines whether to send them to the GA4GH Product Steering Committee (PSC). If so, that committee votes to decide whether GA4GH should pursue product development.
The Product Review Committee forms much earlier in the updated Product Development and Approval Process than it did under the previous process. By introducing outside experts before development starts, GA4GH contributors receive useful feedback and can address potential roadblocks promptly.
The same three members of the Product Review Committee follow the product throughout its life cycle, from study through development, approval, and updates.
Once the Product Steering Committee approves the Problem Statement for development, interested individuals can start working on drafting a technical product, developing tools, or writing frameworks.
The development team — which at this stage often formalises as a product development team within a GA4GH Work Stream — conducts broad community engagement to ground the work in real-world use cases. Team members review progress periodically and meet annually with their Product Review Committee.
Development teams must produce the following outputs, in addition to the product specification itself:
After the development team decides the required outputs are ready, a product can enter the approval phase.
First, the product undergoes an open for comment period. The public, the Product Steering Committee, and anyone who previously noted an interest have at least one month to comment on the group’s outputs.
Once the product group has made revisions based on the public comments, the Product Review Committee, Regulatory & Ethics Work Stream, and Data Security Work Stream provide their feedback to the product group. After another round of revisions, these same three groups decide whether to move the product forward in the process.
If everyone agrees, the product goes to the Product Steering Committee to determine if the content of the product is acceptable. A subset of the committee (including, if necessary, delegates chosen for their domain knowledge) may cast their online ballots over a period of two weeks. If at least 60% of the PSC casts an affirmative ballot, the product is brought to the full Product Steering Committee for a live “due-diligence vote” to ensure the Product Development and Approval Process was followed.
With a “yes” vote at this stage, the product or major version update becomes an official GA4GH product.
The revised Product Development and Approval Process also includes new procedures for making updates to existing products.
When a group decides its product requires an update, members can take one of three paths:
The Product Review Committee approves which path a product team should take.
Urgent fixes result in a fast-tracked patch release. Let’s say you notice a small bug, or a time-sensitive error in documentation recently approved by the Product Steering Committee. The product group should notify the Product Review Committee (PRC) immediately of the issue then start working on a fix. The PRC decides whether the fix is appropriate and ready to be released as a patch.
Continued development results in a minor version release. Examples include fixing trivial problems, clarifying meaning, and adding new tags or terms that fall within the remit that the Product Steering Committee already approved. (No breaking changes are allowed in this path.) Most product maintenance falls under continued development. After the Product Review Committee has reviewed the change, and the public has had one month to comment, an approved minor version can be released.
Scope or breaking changes result in a major version release. Such changes may break backwards compatibility for people already using the product, add significant new features, or take the product in a new direction. To pursue a scope or breaking change, return to study and follow the steps for a new product idea.
Do you want additional information or have a specific question related to your situation? GA4GH staff are here to help. Get in touch!
