About us
Learn how GA4GH helps expand responsible genomic data use to benefit human health.
Learn how GA4GH helps expand responsible genomic data use to benefit human health.
Our Strategic Road Map defines strategies, standards, and policy frameworks to support responsible global use of genomic and related health data.
Discover how a meeting of 50 leaders in genomics and medicine led to an alliance uniting more than 5,000 individuals and organisations to benefit human health.
GA4GH Inc. is a not-for-profit organisation that supports the global GA4GH community.
The GA4GH Council, consisting of the Executive Committee, Strategic Leadership Committee, and Product Steering Committee, guides our collaborative, globe-spanning alliance.
The Funders Forum brings together organisations that offer both financial support and strategic guidance.
The EDI Advisory Group responds to issues raised in the GA4GH community, finding equitable, inclusive ways to build products that benefit diverse groups.
Distributed across a number of Host Institutions, our staff team supports the mission and operations of GA4GH.
Curious who we are? Meet the people and organisations across six continents who make up GA4GH.
More than 500 organisations connected to genomics — in healthcare, research, patient advocacy, industry, and beyond — have signed onto the mission and vision of GA4GH as Organisational Members.
These core Organisational Members are genomic data initiatives that have committed resources to guide GA4GH work and pilot our products.
This subset of Organisational Members whose networks or infrastructure align with GA4GH priorities has made a long-term commitment to engaging with our community.
Local and national organisations assign experts to spend at least 30% of their time building GA4GH products.
Anyone working in genomics and related fields is invited to participate in our inclusive community by creating and using new products.
Wondering what GA4GH does? Learn how we find and overcome challenges to expanding responsible genomic data use for the benefit of human health.
Study Groups define needs. Participants survey the landscape of the genomics and health community and determine whether GA4GH can help.
Work Streams create products. Community members join together to develop technical standards, policy frameworks, and policy tools that overcome hurdles to international genomic data use.
GIF solves problems. Organisations in the forum pilot GA4GH products in real-world situations. Along the way, they troubleshoot products, suggest updates, and flag additional needs.
GIF Projects are community-led initiatives that put GA4GH products into practice in real-world scenarios.
The GIF AMA programme produces events and resources to address implementation questions and challenges.
NIF finds challenges and opportunities in genomics at a global scale. National programmes meet to share best practices, avoid incompatabilities, and help translate genomics into benefits for human health.
Communities of Interest find challenges and opportunities in areas such as rare disease, cancer, and infectious disease. Participants pinpoint real-world problems that would benefit from broad data use.
The Technical Alignment Subcommittee (TASC) supports harmonisation, interoperability, and technical alignment across GA4GH products.
Find out what’s happening with up to the minute meeting schedules for the GA4GH community.
See all our products — always free and open-source. Do you work on cloud genomics, data discovery, user access, data security or regulatory policy and ethics? Need to represent genomic, phenotypic, or clinical data? We’ve got a solution for you.
All GA4GH standards, frameworks, and tools follow the Product Development and Approval Process before being officially adopted.
Learn how other organisations have implemented GA4GH products to solve real-world problems.
Help us transform the future of genomic data use! See how GA4GH can benefit you — whether you’re using our products, writing our standards, subscribing to a newsletter, or more.
Join our community! Explore opportunities to participate in or lead GA4GH activities.
Help create new global standards and frameworks for responsible genomic data use.
Align your organisation with the GA4GH mission and vision.
Want to advance both your career and responsible genomic data sharing at the same time? See our open leadership opportunities.
Join our international team and help us advance genomic data use for the benefit of human health.
Discover current opportunities to engage with GA4GH. Share feedback on our products, apply for volunteer leadership roles, and contribute your expertise to shape the future of genomic data sharing.
Solve real problems by aligning your organisation with the world’s genomics standards. We offer software dvelopers both customisable and out-of-the-box solutions to help you get started.
Learn more about upcoming GA4GH events. See reports and recordings from our past events.
Speak directly to the global genomics and health community while supporting GA4GH strategy.
Be the first to hear about the latest GA4GH products, upcoming meetings, new initiatives, and more.
Questions? We would love to hear from you.
Read news, stories, and insights from the forefront of genomic and clinical data use.
Publishes regular briefs exploring laws and regulations, including data protection laws, that impact genomic and related health data sharing
Translates findings from studies on public attitudes towards genomic data sharing into short blog posts, with a particular focus on policy implications
Attend an upcoming GA4GH event, or view meeting reports from past events.
See new projects, updates, and calls for support from the Work Streams.
Read academic papers coauthored by GA4GH contributors.
Listen to our podcast OmicsXchange, featuring discussions from leaders in the world of genomics, health, and data sharing.
Check out our videos, then subscribe to our YouTube channel for more content.
View the latest GA4GH updates, Genomics and Health News, Implementation Notes, GDPR Briefs, and more.
31 Aug 2022
In situations where data misuse is possible, such as in interactions between patients or publics and genomics researchers, trust is essential. In this guest blog post, the Public Attitudes for Genomic Policy subgroup from the GA4GH Regulatory and Ethics Work Stream delves into the importance of building trust for genomics research.

This guest blog post launches the start of a new series on Public Attitudes for Genomic Policy. This subgroup aims to disseminate the findings from the Your DNA Your Say study and other public attitudes studies into a series of blog posts and accompanying infographics. Touching upon a range of themes, these will inform the Global Alliance for Genomics and Health and external audiences on public attitudes towards genomic research and how policy and research should be designed to address these views.
Genomics research has great potential to contribute to improved human health for all by adding to our understanding of the complexities of the human genome and leading to improved diagnosis, treatments, personalized medicine and public health. The success of genomics relies on (1) access to large and diverse sets of individuals’ genomic and health data, and (2) the ability to share this data with other researchers in some form – potentially internationally. However, in making genomic and other health information available, individuals and communities may become vulnerable to the misuse of these data – whether intentional or not.
In situations where data misuse is possible, such as in interactions between patients or publics and genomics researchers, trust is essential. While definitions of trust vary, for the purposes of this document, we define trust as an attitude that enables an individual to rely on another to take a specific action. It is an attitude that arises within, and is fostered by, meaningful relationships between individuals and communities, and that relates to the perceived qualities of the person or organization being trusted.
Trust is especially essential when those providing data might already be considered vulnerable and when there is uncertainty about the future. In the case of genomics, people cannot generally be informed about all of the potential future uses of their data at the time at which those data are collected, because these uses may currently be unknown to researchers themselves. It may also be difficult to specify at the outset by whom data may be used. The potential negative consequences associated with the misuse of genomic data may be greater for individuals and communities who continue to face historical and contemporary injustices and inequalities associated with genomics, medical research and healthcare. Concerns about the use and potential misuse of data may also arise in relation to some users of data (e.g. commercial entities).
It is thought that public trust is an essential underpinning of willingness to engage with and support genomics initiatives, including donating data and facilitating public funding for genomic research. Where trust is absent, genomics initiatives may struggle to fulfill their potential because they cannot attract public support and engagement or secure future public funding.
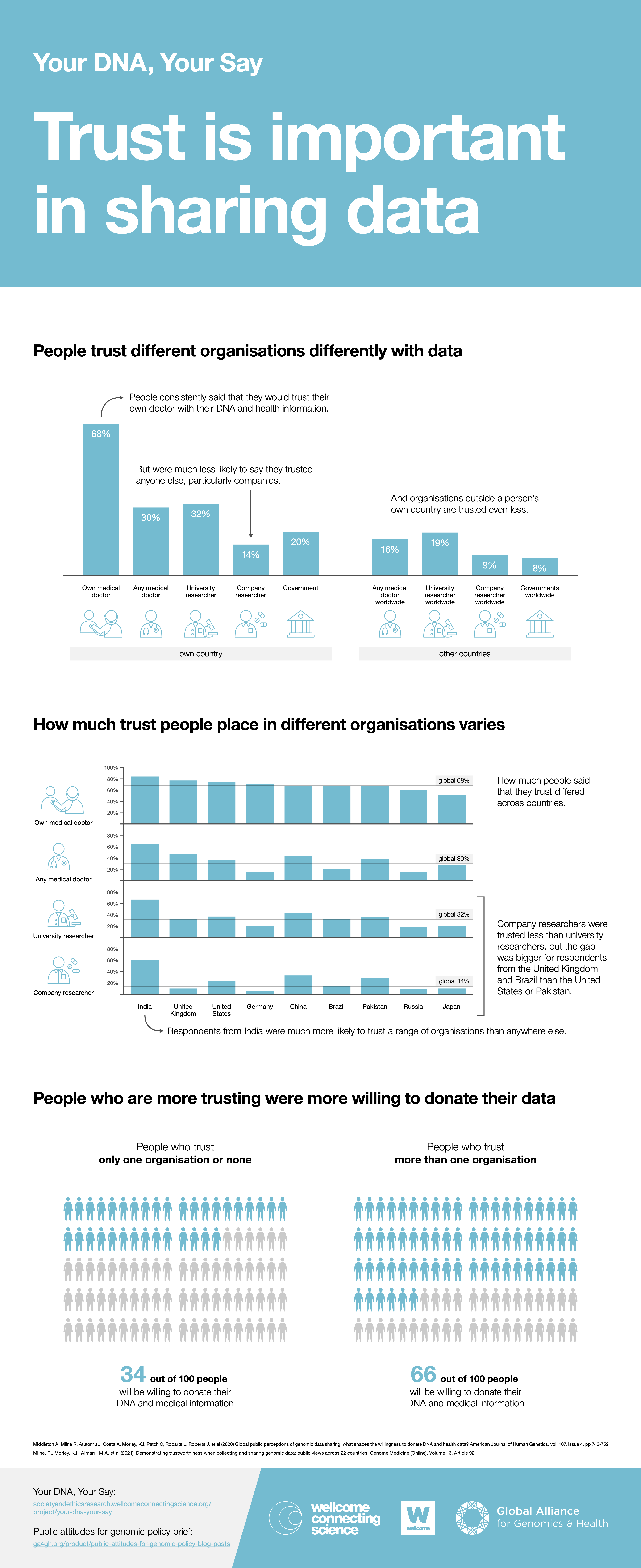
There is a growing literature on the importance and distribution of trust related to the donation of genomic data. In this brief, we concentrate on the findings of Your DNA, Your Say, a questionnaire survey that includes responses from 37,000 individuals across 22 countries. It explores levels of trust in the individuals and institutions collecting genomic and health data. The study found that overall levels of trust are highest among respondents in China, India, the UK and Pakistan and lowest among respondents from Egypt, Russia, Germany and Poland. Among the variety of data stewards presented, personal doctors were consistently the most trusted with DNA and health data. Across the countries, less than half of all respondents report trusting someone other than their own doctor. Respondents generally reported trusting researchers from non-profit research organizations substantially more than researchers from for-profit organizations. It was also found that all organizations within the same country were trusted more than the equivalent organizations outside a respondent’s own country. This reflects previous findings (see for example references 3 and 9) that commercialisation can reduce trust and that trust may decrease when data moves across borders.
In addition, the findings from Your DNA, Your Say demonstrate the importance of trust in individuals’ decisions about donating genomic data, and thus for the future of genomic medicine. Across the study, respondents who trusted more were substantially more likely to be willing to donate their genomic and health data for research than those who trusted less. Those who trusted an organization were also more likely to donate data for use by that organization than those who did not.
While public trust appears to be important, it is less clear how it can be obtained and sustained by genomics initiatives. A more tractable approach is to ask what genomics initiatives can do to be seen as worthy of trust by the wider public? The Your DNA, Your Say study explored a range of options that may foster trust, from transparency about data use, to direct interactions with data gatekeepers, to details about sanctions. It examined how these different options were rated by respondents across the 22 countries studied. The most important thing that respondents said would enable trust was transparent information about who would benefit from data access (the most selected option in 17 countries). This was followed by the option to withdraw data and to be provided with information about who is using data, and for what purpose. In contrast, providing websites about data access, biographies of researchers, and enabling direct communication with gatekeepers were seen as less important. Respondents in a few countries diverged significantly from this general pattern. For example in Japan the most important factor seen as enabling trust was knowing what sanctions would be in place in the case of data misuse.
These variations are, however, of significant potential importance for making policy around the development of genomics initiatives. There are some cases where the strength of variation may flag potential challenges associated with international standard setting and policy making. In the Your DNA, Your Say study, this was the case for responses from China, Japan and Russia, which differed substantially from those of respondents from other countries in the perceived value of measures to enable trust. However, even where variation is less pronounced, policy should be attentive to the local contexts in which trust is established, to the work involved in establishing trusting relations and with historically underserved or excluded communities.
The findings of the Your DNA, Your Say study on public attitudes regarding genomic and health data sharing show that trust is an important factor in the willingness of members of the public to donate DNA and health data. However, respondents’ attitudes of trust varied in relation to different organizations using data. There is also considerable variability in attitudes of trust among respondents from different countries. Doctors are generally trusted the most, commercial entities the least, and data users outside an individuals’ own country trusted less than domestic users. These findings reflect the wider literature on trust.
These findings on trust highlight the importance of building relationships of trust for genomics research. This can help people feel that their trust is justified, and that it is placed in those who are worthy of it. The findings of Your DNA Your Say suggest that important features of demonstrating trustworthiness include being transparent about the goals and benefits associated with genomics, allowing individuals to exert control over the inclusion of their data, and, in some contexts, strong enforcement regimes.

Authors
This brief was prepared by Richard Milne, Maili Raven-Adams, Dianne Nicol and the Public Attitudes for Genomic Policy Subgroup, Regulatory and Ethics Work Stream, GA4GH.
Your DNA, Your Say
More information on the Your DNA, Your Say study can be found here and in the publications below. The full dataset is available for download at https://osf.io/px3gb/
Middleton, Anna, Richard Milne, Mohamed A. Almarri, Shamim Anwer, Jerome Atutornu, Elena E. Baranova, Paul Bevan, et al. “Global Public Perceptions of Genomic Data Sharing: What Shapes the Willingness to Donate DNA and Health Data?” The American Journal of Human Genetics 107, no. 4 (October 1, 2020): 743–52. https://doi.org/10.1016/j.ajhg.2020.08.023.
Milne, Richard, Katherine I. Morley, Heidi Howard, Emilia Niemiec, Dianne Nicol, Christine Critchley, Barbara Prainsack, et al. “Trust in Genomic Data Sharing among Members of the General Public in the UK, USA, Canada and Australia.” Human Genetics 138, no. 11 (December 1, 2019): 1237–46. https://doi.org/10.1007/s00439-019-02062-0.
Milne, Richard, Katherine I. Morley, Mohamed A. Almarri, Shamim Anwer, Jerome Atutornu, Elena E. Baranova, Paul Bevan, et al. “Demonstrating Trustworthiness When Collecting and Sharing Genomic Data: Public Views across 22 Countries.” Genome Medicine 13, no. 1 (May 25, 2021): 92. https://doi.org/10.1186/s13073-021-00903-0.
Further reading
This brief is part of the Public Attitudes for Genomic Policy blog series, developed by the GA4GH Regulatory & Ethics Work Stream (REWS). Visit here to read the past brief.
