About us
Learn how GA4GH helps expand responsible genomic data use to benefit human health.
Learn how GA4GH helps expand responsible genomic data use to benefit human health.
Our Strategic Road Map defines strategies, standards, and policy frameworks to support responsible global use of genomic and related health data.
Discover how a meeting of 50 leaders in genomics and medicine led to an alliance uniting more than 5,000 individuals and organisations to benefit human health.
GA4GH Inc. is a not-for-profit organisation that supports the global GA4GH community.
The GA4GH Council, consisting of the Executive Committee, Strategic Leadership Committee, and Product Steering Committee, guides our collaborative, globe-spanning alliance.
The Funders Forum brings together organisations that offer both financial support and strategic guidance.
The EDI Advisory Group responds to issues raised in the GA4GH community, finding equitable, inclusive ways to build products that benefit diverse groups.
Distributed across a number of Host Institutions, our staff team supports the mission and operations of GA4GH.
Curious who we are? Meet the people and organisations across six continents who make up GA4GH.
More than 500 organisations connected to genomics — in healthcare, research, patient advocacy, industry, and beyond — have signed onto the mission and vision of GA4GH as Organisational Members.
These core Organisational Members are genomic data initiatives that have committed resources to guide GA4GH work and pilot our products.
This subset of Organisational Members whose networks or infrastructure align with GA4GH priorities has made a long-term commitment to engaging with our community.
Local and national organisations assign experts to spend at least 30% of their time building GA4GH products.
Anyone working in genomics and related fields is invited to participate in our inclusive community by creating and using new products.
Wondering what GA4GH does? Learn how we find and overcome challenges to expanding responsible genomic data use for the benefit of human health.
Study Groups define needs. Participants survey the landscape of the genomics and health community and determine whether GA4GH can help.
Work Streams create products. Community members join together to develop technical standards, policy frameworks, and policy tools that overcome hurdles to international genomic data use.
GIF solves problems. Organisations in the forum pilot GA4GH products in real-world situations. Along the way, they troubleshoot products, suggest updates, and flag additional needs.
GIF Projects are community-led initiatives that put GA4GH products into practice in real-world scenarios.
The GIF AMA programme produces events and resources to address implementation questions and challenges.
NIF finds challenges and opportunities in genomics at a global scale. National programmes meet to share best practices, avoid incompatabilities, and help translate genomics into benefits for human health.
Communities of Interest find challenges and opportunities in areas such as rare disease, cancer, and infectious disease. Participants pinpoint real-world problems that would benefit from broad data use.
The Technical Alignment Subcommittee (TASC) supports harmonisation, interoperability, and technical alignment across GA4GH products.
Find out what’s happening with up to the minute meeting schedules for the GA4GH community.
See all our products — always free and open-source. Do you work on cloud genomics, data discovery, user access, data security or regulatory policy and ethics? Need to represent genomic, phenotypic, or clinical data? We’ve got a solution for you.
All GA4GH standards, frameworks, and tools follow the Product Development and Approval Process before being officially adopted.
Learn how other organisations have implemented GA4GH products to solve real-world problems.
Help us transform the future of genomic data use! See how GA4GH can benefit you — whether you’re using our products, writing our standards, subscribing to a newsletter, or more.
Join our community! Explore opportunities to participate in or lead GA4GH activities.
Help create new global standards and frameworks for responsible genomic data use.
Align your organisation with the GA4GH mission and vision.
Want to advance both your career and responsible genomic data sharing at the same time? See our open leadership opportunities.
Join our international team and help us advance genomic data use for the benefit of human health.
Discover current opportunities to engage with GA4GH. Share feedback on our products, apply for volunteer leadership roles, and contribute your expertise to shape the future of genomic data sharing.
Solve real problems by aligning your organisation with the world’s genomics standards. We offer software dvelopers both customisable and out-of-the-box solutions to help you get started.
Learn more about upcoming GA4GH events. See reports and recordings from our past events.
Speak directly to the global genomics and health community while supporting GA4GH strategy.
Be the first to hear about the latest GA4GH products, upcoming meetings, new initiatives, and more.
Questions? We would love to hear from you.
Read news, stories, and insights from the forefront of genomic and clinical data use.
Publishes regular briefs exploring laws and regulations, including data protection laws, that impact genomic and related health data sharing
Translates findings from studies on public attitudes towards genomic data sharing into short blog posts, with a particular focus on policy implications
Attend an upcoming GA4GH event, or view meeting reports from past events.
See new projects, updates, and calls for support from the Work Streams.
Read academic papers coauthored by GA4GH contributors.
Listen to our podcast OmicsXchange, featuring discussions from leaders in the world of genomics, health, and data sharing.
Check out our videos, then subscribe to our YouTube channel for more content.
View the latest GA4GH updates, Genomics and Health News, Implementation Notes, GDPR Briefs, and more.
23 May 2023
Understanding public and other stakeholder attitudes towards analysis and the return of clinically actionable results and other data will assist researchers, research institutions, professional bodies, and policymakers in formulating further best practice guidance and/or regulatory tools. The Your DNA, Your Say (YDYS) study examined whether the return of research results – described in terms of a readout of an individual’s DNA — would influence the willingness of members of the public to donate their DNA data and medical information to research.

The increasing availability and sophistication of genomic analysis is enhancing our understanding of the role of genomic variants in disease, particularly through whole genome and whole exome sequencing. Clinically actionable results, which reveal or predict conditions where preventive measures and/or treatments are available, may be generated as a result of genomic analysis of an individual’s genome, occurring in both clinical or research settings. However, the precise scope of the legal and ethical responsibility to return the results of genome analysis to research participants remains somewhat unclear, and current requirements vary between jurisdictions.
As a starting point, it is helpful to consider how ‘return of results’ is handled in clinical contexts. All clinical genetic testing is primarily designed to detect and return results related to the purpose of testing. Whole genome and whole exome sequencing may also detect clinically actionable genomic variation not otherwise related to the purpose of testing. Under what circumstances, if any, it is permissible, recommended, or mandatory to return such findings in clinical contexts is controversial. For example, the US-based American College of Medical Genetics and Genomics (‘ACMG’) has issued a set of guidelines with a ‘minimum list’ of variants that should be routinely assessed by clinicians on the basis that they have a high degree of clinical validity and utility. However, this approach has not been endorsed by professional bodies in some other jurisdictions.
In the research context, whether and how results should be returned has also been contested. Indeed, a recent review of international, regional and national laws relating to return of research results and incidental findings across 20 countries revealed a wide divergence in approaches. In contrast to these regulatory and legal differences between jurisdictions, there is broad consensus amongst researchers and research participants that return of results, whether related to the purpose of the research study or incidental, is desirable, especially when such findings are actionable. In light of this, in 2021 the Global Alliance for Genomics and Health (GA4GH) developed a policy addressing Clinically Actionable Genomic Research Results, which advocates for return of results to participants, without prescribing which results to return. The H3Africa Consortium has provided further guidance to inform decisions about whether to return individual genetic research findings by Consortium members to research participants.
Understanding public and other stakeholder attitudes towards analysis and the return of clinically actionable results and other data will assist researchers, research institutions, professional bodies, and policy makers in formulating further best practice guidance and/or regulatory tools.
Multiple studies have indicated that research participants have a high interest in receiving their individual research results. Across 221 articles analysed in a recent systematic review, research participants showed high interest in receiving primary research findings (that is, findings directly related to the research question). This was found to be the case particularly when the results were clinically actionable, although the studies analysed revealed more equivocal trends with regard to secondary and incidental findings (referred to by the authors of the systematic review as ‘secondary and unsolicited findings’).
Overall, the authors conclude that the empirical evidence provides strong support, whether from the perspective of the potential recipients of research results or researchers and other stakeholders, for return of research results that are reliable and clinically relevant.
However, most (80%) of the studies identified by the systematic review were undertaken in the US and Canada. Given international variation in both health systems and attitudes to personal data, there remains an open question over whether the outcomes of this extensive body of empirical studies in these jurisdictions, which tend to support the return of research results, is a clear indicator of global attitudes. This points to the need to examine stakeholder views in other, more culturally diverse contexts. Your DNA, Your Say (YDYS) is an attempt to address this need.
YDYS was a questionnaire survey that collected responses between 2017 and 2019. The YDYS dataset includes responses from 37,000 individuals across 22 countries. The study examined whether the return of research results – described in terms of a readout of an individual’s DNA – would influence the willingness of members of the public to donate their DNA data and medical information to research. The study revealed substantial variation in the extent to which return of results influenced willingness to donate across the countries studied.
In 16 of the 21 countries studied in the YDYS survey, respondents were substantially less likely to be motivated by the return of research results to donate DNA or health data than those in the US and more likely to be not influenced by the return of these results at all. This was only countered by respondents from Russia, where participants were likely to be more influenced. Indeed, respondents from across 20 countries had lower odds of being partially or wholly influenced by return of results than those from the US.
Overall, 25.2% of the respondents said they would not donate their DNA and medical information (regardless of return of results); 24.4% of the respondents said their decision to donate would be partially influenced, and 18.4% of the respondents said their decision would be wholly influenced by return of results. A little more than 17% of the respondents reported that they were unsure, whereas 14.7% of the respondents reported that their decision to donate would not be influenced by return of results at all.
Responses relating to willingness to donate show that being wholly influenced by return of results was most commonly reported by respondents from Russia (32.7%), the US (30.5%), India (28%), and Pakistan (26.9%). This was least commonly reported by respondents from Japan (9.2%), Spain (11.6%), Sweden (12.7%), and the UK (13.9%). Being partially influenced by return of results was most commonly reported by respondents from China (39.4%), Poland (27.8%), and Russia (27.6%). Respondents from Brazil, Portugal and Italy were most likely to report that return of results had no influence on their willingness to donate their data (24.1%, 23.7%, and 22.2% respectively). This clearly illustrates that there are differences in public attitudes towards return of research results between countries, and that studies focused wholly on the US and Canada do not reflect this global diversity.
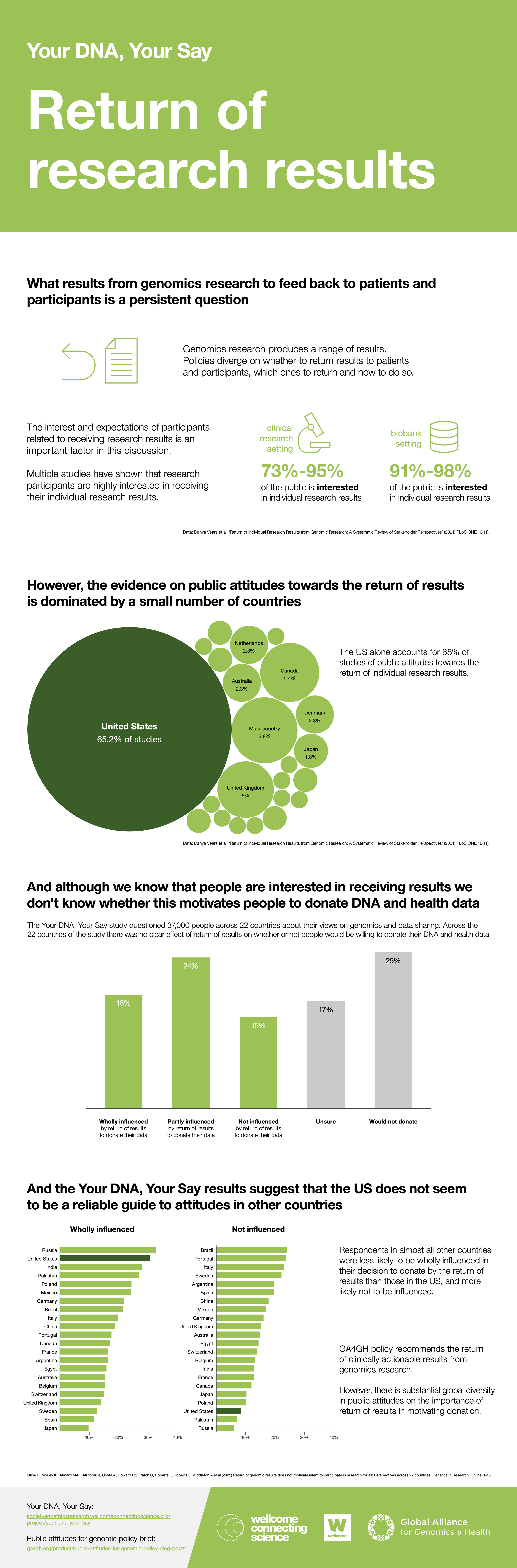
The comparative findings from YDYS illustrate that public attitudes to return of research results are nuanced and heterogeneous. Specifically, the findings suggest that there is a need to distinguish between peoples’ general interest in information about themselves and the expectation that this will be provided as a quid pro quo for data donation, and that the importance of the latter varies across countries.
These findings are important when considering the role of public perspectives in shaping the design of return of results policies, particularly where genomics initiatives contemplate moves beyond the return of narrow predefined results (such as the list proposed by the ACMG) to more open forms of data access. It suggests that while people may be highly interested in receiving research results – and there may be other valid ethical arguments for providing these – it does not necessarily follow that not receiving results would prevent their participation.
The results of the YDYS study suggest that while members of the public may be guided by reciprocity in their decisions about whether or not to share their DNA and medical information, it is not necessarily in the sense of a transactional quid pro quo for participation. This suggests that, rather than a transactional arrangement, the duty of reciprocity should be seen as underpinning the evolution of a robust social contract for genomic medicine into the future.
It should be noted that further research into public attitudes towards return of research results is currently being undertaken in many jurisdictions, using a variety of methods beyond population-wide surveys.
Hoell, Christin, Julia Wynn, Luke V. Rasmussen, Keith Marsolo, Sharon A. Aufox, Wendy K. Chung, John J. Connolly, et al. “Participant Choices for Return of Genomic Results in the EMERGE Network.” Genetics in Medicine 22, no. 11 (November 2020): 1821–29. https://doi.org/10.1038/s41436-020-0905-3.
Middleton, Anna, Katherine I Morley, Eugene Bragin, Helen V Firth, Matthew E Hurles, Caroline F Wright, and Michael Parker. “Attitudes of Nearly 7000 Health Professionals, Genomic Researchers and Publics toward the Return of Incidental Results from Sequencing Research.” European Journal of Human Genetics 24, no. 1 (2016): 21–29. https://doi.org/10.1038/ejhg.2015.58.
Middleton, Anna, Caroline F. Wright, Katherine I. Morley, Eugene Bragin, Helen V. Firth, Matthew E. Hurles, and Michael Parker. “Potential Research Participants Support the Return of Raw Sequence Data.” Journal of Medical Genetics 52, no. 8 (August 1, 2015): 571–74. https://doi.org/10.1136/jmedgenet-2015-103119.
Mwaka, Erisa Sabakaki, Deborah Ekusai Sebatta, Joseph Ochieng, Ian Guyton Munabi, Godfrey Bagenda, Deborah Ainembabazi, David Kaawa-Mafigiri, “Researchers’ Perspectives on Return of Individual Genetics Results To Research Participants: A Qualitative Study” Global Bioethics 32 (2021): 15-33. https://doi.org/10.1080/11287462.2021.1896453
Nobile, Hélène, Pascal Borry, Jennifer Moldenhauer, and Manuela M Bergmann. “Return of Results in Population Studies: How Do Participants Perceive Them?” Public Health Ethics 14, no. 1 (April 1, 2021): 12–22. https://doi.org/10.1093/phe/phaa034.
Sanderson, Saskia C., Michael A. Diefenbach, Randi Zinberg, Carol R. Horowitz, Margaret Smirnoff, Micol Zweig, Samantha Streicher, Ethylin Wang Jabs, and Lynne D. Richardson. “Willingness to Participate in Genomics Research and Desire for Personal Results among Underrepresented Minority Patients: A Structured Interview Study.” Journal of Community Genetics 4 (2013): 469–82.
Sanderson, Saskia C., Michael D. Linderman, Sabrina A. Suckiel, George A. Diaz, Randi E. Zinberg, Kadija Ferryman, Melissa Wasserstein, Andrew Kasarskis, and Eric E. Schadt. “Motivations, Concerns and Preferences of Personal Genome Sequencing Research Participants: Baseline Findings from the HealthSeq Project.” European Journal of Human Genetics 24, no. 1 (January 2016): 14–20. https://doi.org/10.1038/ejhg.2015.118.
Sayeed, Sabina, Robert Califf, Robert Green, Celeste Wong, Kenneth Mahaffey, Sanjiv Sam Gambhir, Jessica Mega, et al. “Return of Individual Research Results: What Do Participants Prefer and Expect?” PLOS ONE 16, no. 7 (July 29, 2021): e0254153. https://doi.org/10.1371/journal.pone.0254153.
Vears, Danya F., Joel T. Minion, Stephanie J. Roberts, James Cummings, Mavis Machirori, Mwenza Blell, Isabelle Budin-Ljøsne, et al. “Return of Individual Research Results from Genomic Research: A Systematic Review of Stakeholder Perspectives.” PLOS ONE 16, no. 11 (November 8, 2021): e0258646. https://doi.org/10.1371/journal.pone.0258646.
Van Der Merwe, Nicole, Raj Ramesar, Jantina De Vries, “Whole Exome Sequencing in South Africa: Stakeholder Views on Return of Individual Research Results and Incidental Findings.” Frontiers of Genetics. 13 (2022): 864822. https://doi.org/10.3389/fgene.2022.864822
Dianne Nicol, Maili Raven-Adams, Richard Milne and the Public Attitudes for Genomic Policy Subgroup, Regulatory and Ethics Work Stream, GA4GH. The YDYS study was designed and led by Anna Middleton.
This brief is part of the Public Attitudes for Genomic Policy blog series, developed by the GA4GH Regulatory & Ethics Work Stream (REWS). Visit here to read all briefs.
